|
|
|
报告导航:研究报告—
生命科学—制药医疗
|
|
2010年中国心血管介入器械行业研究报告 |
 |
字数:2.7万 |
页数:67 |
图表数:62 |
|
中文电子版:5500元 |
中文纸版:2750元 |
中文(电子+纸)版:6000元 |
|
英文电子版:1700美元 |
英文纸版:1800美元 |
英文(电子+纸)版:2000美元 |
|
编号:ZT004
|
发布日期:2011-01 |
附件:下载 |
 |
|
|
近年来,中国心脑血管疾病患病率逐年提高,根据中国卫生部数据,2008年中国冠心病患者约为2000万人,且每年新增超过100万。急剧增长的心脏病患者刺激了中国心脏介入手术和冠脉支架需求的快速增长。中国PCI手术从2002年约2.5万例增长至2009年的24.2万例;冠脉支架植入数量从2002年约4.0万套增长至2009年的38.7万套。2009年中国心血管介入器械行业市场规模(按医院终端销售额计)已达81.6亿元左右,同比增长约30%。 图:2002-2012年中国PCI手术病例数和冠脉支架植入数(单位:千例) 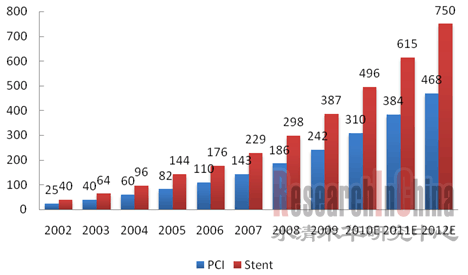 来源:中国医疗器械行业协会,水清木华研究中心 心血管介入器械行业包括冠脉支架和手术配套器械,2009年中国冠脉支架市场规模约57.1亿元,以球囊导管、导管、导丝等组成的手术配套器械市场规模24.6亿元,两者市场规模比例约为7:3。 在冠脉支架市场,2004年以前,中国冠脉支架市场主要被外资企业占据,随着中国企业对核心技术的不断探索和积累,依靠价格和渠道优势,国产冠脉支架的市场占有率逐年增加并远超外资企业。从植入数量上看,2009年国内企业约占77.7%的市场份额,外资企业约占22.3%;从医院终端销售额上看,2009年国内企业约占66.8%的市场份额,外资企业约占33.2%。 图:2009年中国冠脉支架市场份额(以医院销售额计) 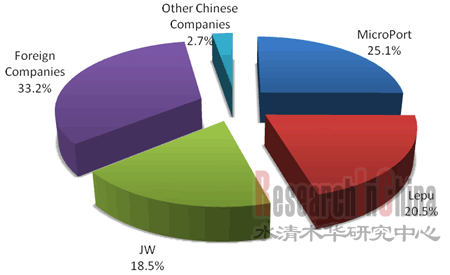 来源:微创招股说明书,水清木华研究中心 2009年,冠脉支架市场份额居于前三位的企业分别是微创医疗、乐普医疗和吉威医疗,按医院终端销售额计,三者市场份额占比分别为25.1%、20.5%和18.5%。其中,微创和乐普公司心血管介入器械产品线比较丰富,并且这两家公司的发展计划均是冠脉支架为主,造影导管、导引导丝、鞘组等等其他心血管介入相关产品为辅,逐渐覆盖整个心血管介入器械领域。此外,两家公司均在不断加大心血管介入器械的研发力度,如微创正在研发第三代药物洗脫支架Firehawk,乐普正在研发新一代的镁合金可降解支架。 但是,在球囊导管、导管、导丝等组成的手术配套器械市场,受技术限制,中国国产化率不到20%,基本依赖进口,整个市场主要被强生Cordis、美敦力、波士顿等外资品牌占据。然而这种局面也给中国企业创造了复制冠脉支架发展之路的机会,微创、乐普、福基阳光、合众赛富等企业正加大对此类产品的研发和生产力度,未来心血管介入配套器械市场的竞争将会加剧。 《2010年中国心血管介入器械行业研究报告》立足于中国心血管介入器械行业的国内外社会、经济和政策环境,行业整体运行状况,市场竞争格局和发展趋势,在重点研究了中国冠脉支架、球囊导管、导管、导丝、辅助装置等心血管介入器械细分市场的同时,还着重分析了中国市场上12家主要心血管介入器械制造企业(包括强生J&J Cordis、美敦力、波士顿科学、雅培概腾、贝朗、微创、乐普、吉威、垠艺、业聚、福基阳光、益心达)的经营和发展情况。
In recent years, the prevalence rate of cardiovascular disease has
increased year by year. According to the data issued by Ministry of
Health of the People’s Republic of China, there were 20 million patients
with coronary artery disease in China 2008, and the number rises at the
annual growth of more than 1 million. The growth spurt of the patients
suffering from heart disease has stimulated the fast growing demand for
interventional cardiology operations and coronary stents. In
China, the number of PCI operation cases rose from 25,000 in 2002 to
242,000 in 2009; the coronary stent implantation amount increased from
40,000 sets in 2002 to 387,000 in 2009. In 2009, Chinese market size of
interventional cardiovascular device (by terminal sales revenue of
hospitals) reached RMB8.16 billion, up 30% from a year earlier. PCI Operation Cases and Coronary Stent Implantation Amount in China, 2002-2012 (Unit: 1,000 cases) 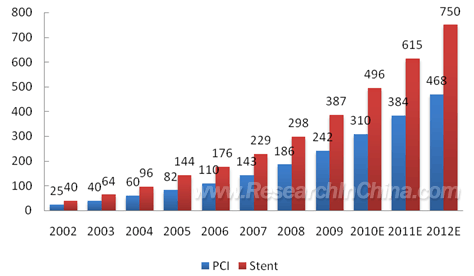 Source: China Association for Medical Devices Industry, ResearchInChina Interventional
cardiovascular device includes coronary stent and surgical support
equipment. In 2009, Chinese coronary stent market valued RMB5.71
billion; and the market size of surgical support equipment (including
balloon catheter, catheter, and guide wire) hit RMB2.46 billion. The
ratio of the two markets was about 7:3. Before 2004, foreign
companies dominated Chinese coronary stent market. Because of
incessantly exploring core technologies and accumulating experience,
Chinese enterprises enjoy the rising market shares yearly and even more
than foreign counterparts by virtue of advantages in price and channel.
By the amount of implantation, domestic enterprises swept about 77.7%
market shares in 2009, while foreign peers took approximately 22.3%; by
the terminal sales revenue of hospitals, domestic enterprises held
around 66.8% market shares in 2009, while foreign ones occupied 33.2% or
so. Coronary Stent Market Shares in China (by Sale of Hospitals), 2009 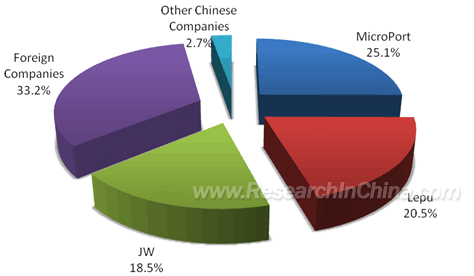 Source: MicroPort, ResearchInChina In
2009, MicroPort Scientific, Lepu Medical and JW Medical ranked the top
three in terms of the market share of coronary stent; by the terminal
sales revenue of hospitals, MicroPort Scientific, Lepu Medical and JW
Medical took the market share of 25.1%, 20.5% and 18.5% respectively.
Remarkably, MicroPort and Lepu are with the diversified product lines of
interventional cardiovascular device, and both of them focus on
coronary stent and also pay attention to angiography catheter,
guidewire, sheath introducer kit, etc, with the objective of ultimately
covering the entire field of interventional cardiovascular devices. In
addition, MicroPort and Lepu are making more efforts in the R&D of
interventional cardiovascular devices. MicroPort is now developing the
third-generation drug-eluting stent “Firehawk” , while Lepu is
developing a new generation of magnesium alloy biodegradable stents. However,
in surgical supporting device market consisting of balloon catheter,
catheter and guidewire, due to technical limitations, only less than 20%
of products are made in China. The market is dominated by Cordis, J
& J, Medtronic, Boston and other foreign brands. Yet, Chinese
enterprises may simulate the development modes of those foreign
companies. MicroPort, Lepu, Fisuntech, TemMed and other companies are
enhancing R&D and production of surgical supporting instruments. In
the future, the competition in the market of interventional
cardiovascular supporting devices will intensify. The report is
based on social, economic and policy environments, the overall
operation, market competition and development trends of Chinese
interventional cardiovascular device industry. It not only studies such
market segments as coronary stents, balloon catheters, catheters,
guidewires, assistive devices and others in China, but also analyzes the
operation and development of 12 major interventional cardiovascular
device manufacturers including J & J Cordis, Medtronic, Boston
Scientific, Abbott Guidant, B. Braun, MicroPort, Lepu, JW, Yinyi,
OrbusNeich, Fisuntech, SCW Medical.
第一章 心血管介入器械行业概述
1.1 定义
1.2 产品种类及应用
1.3 产业链
第二章 中国心血管介入器械行业发展情况
2.1 国际环境
2.2 行业概况
2.3 市场供需
2.4市场规模及毛利
2.5 竞争格局
2.6 相关政策
2.6.1 法律法规
2.6.2 行业发展政策
2.6.3 行业销售政策
2.7 进口情况
2.8 发展趋势
2.8.1 医疗改革提升消费能力
2.8.2企业产品线多元化
第三章 心血管介入细分市场分析
3.1 冠脉支架
3.1.1 市场概况
3.1.2 药物洗脱支架
3.1.3 裸金属支架
3.1.4 生物可吸收支架
3.2 球囊导管
3.2.1 市场概况
3.2.2 供需情况
3.3 导管
3.3.1 市场概况
3.3.2 销售情况
3.4 导丝
3.4.1 市场概况
3.4.2 销售情况
3.5 鞘组和辅助装置
3.5.1 市场概况
3.5.2 销售情况
第四章 国外生产企业分析
4.1 强生Cordis
4.1.1 公司概况
4.1.2 经营情况
4.1.3在华经营情况
4.2美敦力
4.2.1 公司概况
4.2.2 经营情况
4.2.3 在华经营情况
4.3波士顿科学
4.3.1 公司概况
4.3.2 经营情况
4.3.3 在华经营情况
4.4 雅培概腾
4.4.1 公司概况
4.4.2 经营情况
4.4.3 在华经营情况
4.5 贝朗
4.5.1 公司概况
4.5.2 经营情况
4.5.3 在华经营情况
第五章 国内生产企业分析
5.1 微创医疗
5.1.1公司概况
5.1.2 经营情况
5.1.3 业务发展
5.2 乐普医疗
5.2.1 公司概况
5.2.2 经营情况
5.1.3 业务发展
5.3 吉威医疗
5.3.1 公司概况
5.3.2 经营情况
5.3.3 业务发展
5.4大连垠艺
5.4.1 公司概况
5.4.2 经营情况
5.4.3 业务特色
5.5 深圳业聚
5.5.1 公司概况
5.5.2 经营情况
5.5.3 业务特色
5.6 北京福基阳光
5.6.1 公司概况
5.6.2 经营情况
5.6.3 业务发展
5.7 深圳益心达
5.7.1 公司概况
5.7.2 经营状况
5.7.3 业务发展
1 Overview of Interventional Cardiovascular Device Industry
1.1 Definition
1.2 Product Category and Application
1.3 Industry Chain
2 Development of Interventional Cardiovascular Device Industry in China
2.1 International Environment
2.2 Industry Overview
2.3 Supply and Demand
2.4 Market Scale and Gross Profit
2.5 Competition Pattern
2.6 Related Policies
2.6.1 Laws and Regulations
2.6.2 Development Policies
2.6.3 Sales Policies
2.7 Import
2.8 Development Trends
2.8.1 Health Care Reform Improves People’s Spending Power
2.8.2 Diversification of Product Lines
3 Interventional Cardiovascular Market Segments
3.1 Coronary Stent
3.1.1 Market Overview
3.1.2 Drug-eluting Stent
3.1.3 Bare Metal Stent
3.1.4 Bioabsorbable Stent
3.2 Balloon Catheter
3.2.1 Market Overview
3.2.2 Supply & Demand
3.3 Catheter
3.3.1 Market Overview
3.3.2 Sale
3.4 Guidewire
3.4.1 Market Overview
3.4.2 Sale
3.5 Sheath Introducer Kit and Auxiliary Device
3.5.1 Market Overview
3.5.2 Sale
4 Foreign Production Enterprises
4.1 Cordis, J&J
4.1.1 Profile
4.1.2 Operation
4.1.3 Operation in China
4.2 Medtronic
4.2.1 Profile
4.2.2 Operation
4.2.3 Operation in China
4.3 Boston Scientific
4.3.1 Profile
4.3.2 Operation
4.3.3 Operation in China
4.4 Guidant , Abbott
4.4.1 Profile
4.4.2 Operation
4.4.3 Operation in China
4.5 B.Braun
4.5.1 Profile
4.5.2 Operation
4.5.3 Operation in China
5 Chinese Production Enterprises
5.1 MicroPort Scientific
5.1.1Profile
5.1.2 Operation
5.1.3 Development
5.2 Lepu Medical
5.2.1 Profile
5.2.2 Operation
5.1.3 Development
5.3 JW Medical
5.3.1 Profile
5.3.2 Operation
5.3.3 Development
5.4 Yinyi
5.4.1 Profile
5.4.2 Operation
5.4.3 Features
5.5 OrbusNeich
5.5.1 Profile
5.5.2 Operation
5.5.3 Features
5.6 Sinomed
5.6.1 Profile
5.6.2 Operation
5.6.3 Development
5.7 SCW Medical
5.7.1 Profile
5.7.2 Operation
5.7.3 Development
表:心血管介入器械种类及功能
表:心血管支架种类及特点
图:中国心血管介入器械行业产业链
图:2005-2012年全球心脏介入器械市场规模
图:2009年全球主要品牌冠脉支架销售额
图:2002-2009年中国医疗器械市场规模及增长率
图:2005-2010年中国医院总数和增长率
表:中国主要心血管介入器械企业
图:2010年全球部分国家65岁或以上人口数及比例
图:1993-2008年中国心脏病和高血压患病率
图:2002-2012年中国PCI手术病例数
图:2002-2012年中国冠脉支架植入数
图:2009年中国心血管介入器械市场规模
表:2007-2009年中国主要心血管介入器械生产商毛利率
图:2009年中国冠脉支架市场份额(以植入数和医院销售额计)
表:中国有关医疗器械行业的主要法律、法规
表:心血管介入器械行业相关政策
图:中国心血管介入器械销售渠道示意图
图:2008-2010年卫生部集中采购国产和进口冠脉支架均价比较
表:2008年中国主要介入医疗器械及材料进口情况
表:2010年中国京沪穗三城市介入手术医保支付比例
图:2000-2009年中国城镇医疗保险人数及增长率
表:微创、乐普及吉威医疗器械产品线布局情况
图:2007-2015年中国冠脉支架市场规模
图:2009年中国冠脉支架市场份额(以植入支架数量计)
图:2009年中国冠脉支架市场份额(以医院销售额计)
表:2010年中国市场药物洗脱支架产品列表
表:2010年中国市场部分裸金属支架产品列表
表:2002-2009年中国冠脉球囊导管需求量
表:2010年中国市场各类球囊导管采购均价(单位:人民币)
表:2010年中国市场部分快速交换扩张球囊导管产品列表
表:2010年中国市场各类导管采购均价
表:2010年中国市场导引导管产品列表
表:2010年中国市场各类导丝采购均价
表:2010年中国市场部分导引导丝产品列表
表:2010年中国市场鞘组和辅助装置采购均价
图:2007-2010年强生医疗器械收入和Cordis收入及占比
图:2007-2011财年美敦力心血管产品收入、冠脉支架收入及占比
表:2007-2008年美敦力(上海)主要经营指标
图:2008-2010年波士顿科学总收入和冠脉支架收入及占比
图:2008-2010年雅培血管器械部门收入和增长率
图:2006-209年贝朗蛇牌收入和增长率
表:2008年贝朗医疗(苏州)有限公司主要经营指标
图:2007-2010年微创医疗营业收入、毛利率及收入增长率
图:2007-2010年微创医疗(分产品)收入及占比情况
图:2007-2010年微创医疗(分地区)收入及占比情况
图:2007-2010年微创医疗研发费用及占销售额比例
表:截至2010年9月微创医疗新品研发情况
图:2007-2010年微创医疗支架及导管产能、实际产量及利用率
图:2007-2010年乐普医疗营业收入、毛利率及收入增长率
表:2007-2010年乐普医疗(分产品)销售毛利率变动情况
图:2008-2010年乐普医疗(分产品)收入及占比情况
图:2010年乐普医疗(分地区)收入占比情况
图:2007-2009年乐普医疗研发费用及占销售额比例
表:乐普医疗研发布局
图:2007-2008年吉威医疗营业收入及毛利率
表:2008年辽宁生物医学材料主要经营指标
图:2005-2008年深圳业聚营业收入、毛利率及收入增长率
图:2005-2008年深圳业聚出口值
表:2008年福基阳光主要经营指标
图:2005-2008年深圳益心达营业收入、毛利率及收入增长率
图:2005-2008年深圳益心达出口值
Types and Functions of Interventional Cardiovascular Devices
Types and Characteristics of Cardiovascular Stents
China Interventional Cardiovascular Device Industry Chain
Global Market Scale of Interventional Cardiology Devices, 2005-2012
Coronary Stent Sales Volume of Major Brands in the World, 2009
Scale and Growth Rate of Chinese Medical Device Market, 2002-2009
Total Number and Growth Rate of Hospitals in China, 2005-2010
Main Interventional Cardiovascular Device Companies in China
Number of People Aged 65 or above and Proportion in Some Countries, 2010
Prevalence of Heart Disease and Hypertension in China, 1993-2008
Number of PCI Operation Cases in China, 2002-2012
Coronary Stent Implantation Number in China, 2002-2012
Interventional Cardiovascular Device Market Size in China, 2009
Gross Margins of Major Interventional Cardiovascular Device Manufacturers in China, 2007-2009
Coronary Stent Market Shares in China (by Implantation Volume and Sales of Hospitals), 2009
Major Laws and Regulations concerning Medical Device Industry in China
Policies Related to Interventional Cardiovascular Device Industry
Sales Channels of Interventional Cardiovascular Devices in China
Average Price Comparison bettwen Coronary Stents Procured by Ministry of Health of the P.R.C and the Imported Ones, 2008-2010
Import of Major Interventional Medical Devices and Materials in China, 2008
Charges of Interventional Operations paid by Health Insurance in Beijing, Shanghai and Guangzhou, 2010
Urban Health Insurance Participants and Growth Rate in China, 2000-2009
Equipment Product Line Layout of MicroPort, Lepu and JW Medical
Coronary Stent Market Scale in China, 2007-2015
Coronary Stent Market Shares in China (by Volume of Implanted Stents), 2009
Coronary Stent Market Shares in China (by Sale of Hospitals), 2009
Drug-eluting Stents in China, 2010
Some Bare Metal Stents in China, 2010
Demand for Coronary Artery Balloon Catheters in China, 2002-2009
Average Purchase Price of Balloon Catheters in China, 2010
Some Rapid Exchange Expansion Balloon Catheters in China, 2010
Average Purchase Price of Catheters in China, 2010
Guide Catheters in China, 2010
Average Purchase Price of Guidewires in China, 2010
Some Guidewires in China, 2010
Average Purchase Price of Sheath Introducer Kit and Auxiliary Equipment in China, 2010
Medical Device Revenue, Cordis Revenue and Proportion of Johnson & Johnson, 2007-2010
Cardiovascular Product Revenue, Coronary Stent Revenue and Proportion of Medtronic, FY2007-FY2011
Main Operating Indicators of Medtronic (Shanghai), 2007-2008
Total Revenue, Coronary Stent Revenue and Proportion of Boston Scientific, 2008-2010
Revenue and Growth Rate of of Abbott's Vascular Device Sector, 2008-2010
Revenue and Growth Rate of of B. Braun Aesculap, 2006-2009
Main Operating Indicators of B.Braun (Suzhou), 2008
Operating Income, Gross Margin and Revenue Growth Rate of MicroPort Scientific, 2007-2010
Revenue (by Product) of MicroPort Scientific, 2007-2010
Revenue (by Region) of MicroPort Scientific, 2007-2010
R&D Expenditure and Its proportion in Sales Revenue of MicroPort Scientific, 2007-2010
R &D of New Products of MicroPort Scientific, by Sep 2010
Capacity, Actual Output and Utilization of Stents and Catheters of MicroPort Scientific, 2007-2010
Operating Income, Gross Margin and Revenue Growth Rate of Lepu Medical, 2007-2010
Sales Gross Margin (by Product) of Lepu Medical, 2007-2010
Revenue (by Product) of Lepu Medical, 2008-2010
Revenue (by Region) of Lepu Medical, 2010
R &D Expenditure and Its proportion in Sales Revenue of Lepu Medical, 2007-2009
R&D Layout of Lepu Medical
Operating Income and Gross Margin of JW Medical, 2007-2008
Main Operating Indicators of Liaoning Biomedical Materials, 2008
Operating Income, Gross Margin and Revenue Growth Rate of OrbusNeich, 2005-2008
Export Value of OrbusNeich, 2005-2008
Main Operating Indicators of Sinomed, 2008
Operating Income, Gross Margin and Revenue Growth Rate of SCW Medical, 2005-2008
Export Value of SCW Medical, 2005-2008
如果这份报告不能满足您的要求,我们还可以为您定制报告,请 留言说明您的详细需求。
|