|
|
|
报告导航:研究报告—
生命科学—制药医疗
|
|
2011-2012年中国诊断试剂行业研究报告 |
 |
字数:2.4万 |
页数:60 |
图表数:56 |
|
中文电子版:6500元 |
中文纸版:3250元 |
中文(电子+纸)版:7000元 |
|
英文电子版:1400美元 |
英文纸版:1500美元 |
英文(电子+纸)版:1700美元 |
|
编号:HM007
|
发布日期:2011-10 |
附件:下载 |
 |
|
|
诊断试剂分为体外诊断试剂和体内诊断试剂,体外诊断试剂在中国市场所占的比重一直较大,2010年体外诊断试剂占诊断试剂总体市场比重为92.6%。体外诊断试剂行业在中国起步较晚,但受益于中国医疗消费水平的提高以及国家政策的扶持等,体外诊断试剂行业发展迅速。 2010年中国体外诊断行业市场规模为121亿元,2007-2010年复合增长率达13.8%,高于全球体外诊断试剂同期12.6%增速。并且,鉴于中国的人口基数大和经济水平提高等因素,中国体外诊断试剂市场未来增长潜力巨大,预计2014年中国体外诊断试剂市场规模可达232亿元。
图:2007-2014年中国体外诊断行业市场规模及增长率 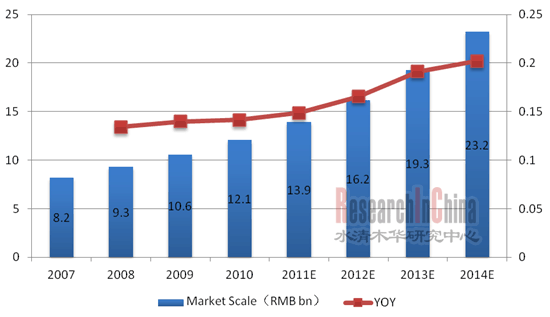 整理:水清木华研究中心 体外诊断产品主要包括诊断试剂和诊断仪器,2010年中国体外诊断试剂市场规模为97亿元,同比增长13.3%,占据体外诊断行业80.2%的比重。规模大的诊断试剂生产企业,往往也是大型的医疗检验仪器生产商,但中国很多企业只生产诊断试剂。中国高端诊断仪器市场被国外品牌占据,如日立、东芝、Olympus、西斯美康等。不过科华生物近年来在诊断仪器方面发展较快,已成为中国诊断试剂加仪器一体化的本土龙头企业。 就细分市场而言,目前中国体外诊断试剂还集中在比较低端的临床免疫和临床化学诊断试剂,2010年市场规模分别为34和31亿元,占中国体外诊断试剂市场的比重分别为35%和32%,近几年伴随着核酸血筛试点的启动,核酸诊断市场进一步扩容,截止2010年年底已有6家药厂向国家食品药品监督管理局(SFDA)申报核酸血筛试剂,即科华生物、达安基因、浩源生物、匹基生物(后被凯杰生物收购)、罗氏和诺华诊断,这些企业将在核酸血筛试点和后继核酸血筛销售中受益。
Diagnostic reagent can be divided into in vitro diagnostic (IVD) reagent and in vivo diagnostic reagent, wherein the former always holds a larger market share in China, accounting for 92.6% in the overall market for diagnostic reagents in 2010. Despite a late start, China’s IVD reagent industry has achieved fast development, benefiting from the improved medical care spending, support of national policies, etc. The market size of China’s IVD industry reached RMB12.1 billion in 2010, with the compound annual growth rate of up to 13.8% from 2007 to 2010, higher than the global growth of 12.6% over the corresponding period. Moreover, considering the large population base, improved economy, etc., China’s IVD reagent industry possesses huge potential for future growth, which is expected to hit RMB23.2 billion in 2014.
Market Size and Growth Rate of In Vitro Diagnostic Industry in China, 2007-2014E 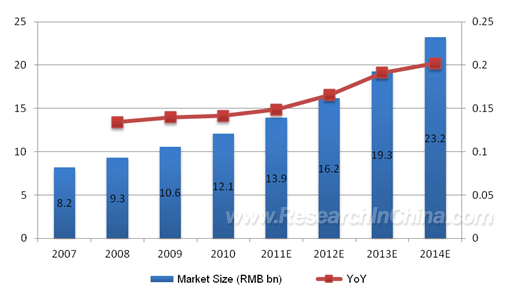 Source: ResearchInChina IVD products mainly consist of diagnostic reagents and instruments. In 2010, the market size of IVD reagents climbed 13.3% YoY to RMB9.7 billion in China, occupying 80.2% of the IVD industry. Large-scale diagnostic reagent manufacturers are often good-sized medical test equipment producers as well, but many Chinese enterprises only deal with diagnostic reagents. In China, the high-end diagnostic instrument market is taken over by foreign brands, such as Hitachi, Toshiba, Olympus, Sysmex, etc. However, after years of rapid improvement in the field of diagnostic instrument, Kehua Bio-engineering (KHB) has become a leading domestic enterprise of diagnostic reagent and instrument. As far as market segment is concerned, China’s IVD reagents are still focused on lower-end clinical immunology and clinical chemistry diagnostic reagents for now, with separate market sizes up to RMB3.4 billion and RMB3.1 billion in 2010, taking 35% and 32% of China’s IVD reagent market respectively. In recent years, along with the launch of nucleic acid blood screening pilot, nucleic acid diagnostics market has been further expanded, as of the end of 2010, there had already been six pharmaceutical factories to declare nucleic acid blood screening reagents to the State Food and Drug Administration (SFDA), namely, KHB, Da An Gene, Haoyuan Biotech, PG Biotech (later acquired by QIAGEN), Roche and Novartis Diagnostics, which will benefit from the nucleic acid blood screening pilot and subsequent marketing.
第一章 诊断试剂行业概述
1.1诊断试剂定义及分类
1.2诊断试剂产业链
1.2.1 上游市场
1.2.2 下游市场
第二章 中国诊断试剂运行环境分析
2.1国际市场分析
2.1.1全球生物制药行业发展情况
2.1.2全球诊断试剂发展情况
2.2中国市场分析
2.2.1中国生物制药发展概况
2.2.2国家相关政策
第三章 中国诊断试剂行业状况
3.1发展现状
3.2 市场规模
3.3 竞争格局
3.4进出口分析
3.4.1 进口分析
3.4.2 出口分析
3.5 诊断试剂细分市场
3.5.1生化诊断试剂
3.5.2免疫诊断试剂
3.5.3核酸诊断试剂
3.6中国诊断试剂发展趋势
第四章 国内主要厂商
4.1科华生物
4.1.1公司介绍
4.1.2经营状况
4.1.3公司诊断试剂经营状况
4.1.4 发展前景及动态
4.2达安基因
4.2.1公司介绍
4.2.2经营状况
4.2.3公司诊断试剂经营状况
4.2.4 发展前景及动态
4.3上海复星医药
4.3.1公司介绍
4.3.2经营状况
4.3.3发展前景及动态
4.3.4上海复星长征医学科学有限公司
4.3.5 上海复星医学科技发展有限公司
4.4中生北控
4.4.1公司介绍
4.4.2经营状况
4.4.3 发展前景及动态
4.5金豪制药
4.5.1公司介绍
4.5.2经营状况
4.5.3发展前景及动态
4.6丽珠试剂
4.6.1公司介绍
4.6.2经营状况
4.7艾康生物
4.7.1公司介绍
4.7.2经营状况
4.8北京利德曼生化技术有限公司
4.8.1公司介绍
4.8.2经营状况
4.8.3 发展前景及动态
4.9万泰生物
4.10上海荣盛生物药业有限公司
4.11深圳匹基
4.11.1公司介绍
4.11.2公司主要产品
4.12上海奥普生物医药有限公司
4.13蓝十字生物药业(北京)有限公司
4.14上海英伯肯医学生物技术有限公司
4.15贝索生物
4.16北方生物
4.17波生生物
1. Industry Profile 1.1 Definition and Classification 1.2 Industrial Chain 1.2.1 Upstream Market 1.2.2 Downstream Market
2. Operating Environment in China
2.1 Global Market
2.1.1 Development of Biopharmaceutical Industry Worldwide
2.1.2 Development of Diagnostic Reagent Industry Worldwide
2.2 Chinese Market
2.2.1 Development of Biopharmaceutical Industry in China
2.2.2 Relevant Policies of China
3. Development in China
3.1 Status Quo
3.2 Market Size
3.3 Competition Pattern
3.4 Import & Export
3.4.1 Import
3.4.2 Export
3.5 Market Segments
3.5.1 Biochemistry Diagnostic Reagent
3.5.2 Immune Diagnostic Reagent
3.5.3 Nucleic Acid Diagnostic Reagent
3.6 Development Tendency of Diagnostic Reagent Sector in China
4. Major Domestic Producers
4.1 Shanghai Kehua Bio-Engineering Co., Ltd. (KHB)
4.1.1 Profile
4.1.2 Operation
4.1.3 Operation of Diagnostic Reagent Business
4.1.4 Prospects and Dynamics
4.2 Da An Gene Co., Ltd. of Sun Yat-Sen University
4.2.1 Profile
4.2.2 Operation
4.2.3 Operation of Diagnostic Reagent Business
4.2.4 Prospects and Dynamics
4.3 Fosun Pharmaceutical (Group) Co., Ltd.
4.3.1 Profile
4.3.2 Operation
4.3.3 Prospects and Dynamics
4.3.4 Shanghai Fosun Long March Medical Science Co., Ltd.
4.3.5 Shanghai Fosun Med-Tech Development Co., Ltd.
4.4 Biosino Bio-Technology & Science Inc.
4.4.1 Profile
4.4.2 Operation
4.4.3 Prospects and Dynamics
4.5 Beijing Kinghawk Pharmaceutical Co., Ltd.
4.5.1 Profile
4.5.2 Operation
4.5.3 Prospects and Dynamics
4.6 Zhuhai Livzon Diagnostics Inc.
4.6.1 Profile
4.6.2 Operation
4.7 ACON Biotech (Hangzhou) Co., Ltd.
4.7.1 Profile
4.7.2 Operation
4.8 Beijing Leadman Biochemistry Co., Ltd
4.8.1 Profile
4.8.2 Operation
4.8.3 Prospects and Dynamics
4.9 Beijing Wantai Biological Pharmacy Enterprise Co., Ltd.
4.10 Shanghai Rongsheng Biotech Co., Ltd.
4.11 Shenzhen PG Biotech Co., Ltd.
4.11.1 Profile
4.11.2 Major Products
4.12 Shanghai Upper Bio-Tech Pharma Co., Ltd
4.13 Blue Cross Bio-Medical (Beijing) Co., Ltd.
4.14 Inverness Medical (Shanghai) Co., Ltd.
4.15 Baso Diagnostics Inc.
4.16 Beijing North Institute of Biological Technology
4.17 Xiamen Boson Biotech Co., Ltd.
图:2007-2012年全球生物制药市场规模
图:2009年全球生物制药(分地区)收入占比
图:2008-2012年全球体外诊断产业市场规模
图:2005-2011年1-5月中国生物生化制药销售收入及同比增长率
表:中国诊断试剂行业相关政策
图:2010年中国诊断试剂行业产品结构
图:2010年中国体外诊断试剂构成
图:2007-2014年中国体外诊断行业市场规模及增长率
图:2007-2014年中国体外诊断试剂市场规模及增长率
表:中国IVD产品主要生产企业
图:2007-2011年1-8月中国X光检查造影剂;用于病人的诊断试剂进口金额、数量及平均价格
表:2011年1-8月中国X光检查造影剂;用于病人的诊断试剂累计(分国别)进口量、进口金额及占比
图:2008-2011年1-8月中国X光检查造影剂(用于病人的诊断试剂)进口目的地分布
图:2007-2011年1-8 月中国X光检查造影剂(用于病人的诊断试剂)出口金额、数量及平均价格表: 2011年1-8月中国X光检查造影剂(用于病人的诊断试剂)累计分国别出口量、出口金额及占比
表:2011年1-8月中国X光检查造影剂(用于病人的诊断试剂)出口货源地分布
图:2007-2014年中国生化试剂市场规模
图:2007-2014年中国免疫试剂市场规模
图:2006-2011H1科华生物营业收入和净利润
图:2007-2011H1科华生物(分产品)营业收入
图:2008-2011H1科华生物(分产品)毛利率
图:2008-2011H1科华生物主营构成(分地区)占比
表:科华生物诊断试剂产品介绍
图:2011-2013年科华生物营业收入与毛利率预测
图:2006-2011H11达安基因营业收入和净利润
图:2007-2011H1达安基因(分产品)营业收入
图:2008-2011H1达安基因(分产品)毛利率
图:2008-2011H1达安基因主营构成(分地区)占比
图:2008-2010年达安基因研发投入及占营业收入比重
图:2011-2013年达安基因营业收入与毛利率预测
图:2006-2011H1复星医药营业收入和净利润
图:2011-2013年复星医药营业收入与毛利率预测
图:2008-2009年复星长征经营情况
图:2008-2009年复星科技经营情况
图:2006-2011H1年中生北控营业收入和净利润
图:2008-2011H1中生北控(分产品)营业收入
表:2010年中生北控获得体外诊断试剂产品证书及申请专利
图:2008-2011H1金豪制药营业收入和净利润
图:2010-2011H1金豪制药(分产品)营业收入
图:2010-2011H1金豪制药(分地区)营业收入
图:2007-2011H1丽珠试剂营业收入
表:2010年6月-2011年9月丽珠试剂所获注册证书
图:2008-2009年艾康生物经营情况
表:2010年3月和2011年5月艾康生物所获医疗器械注册证书
图:2008-2011年北京利德曼体外诊断试剂产能、产量及销售(单位:千升)
表:2010-2011年北京利德曼(分产品)体外诊断试剂产销量(单位:升)
图:2008-2011年北京利德曼体外诊断试剂销售收入及其占比(单位:百万元)
表:2008-2011年北京利德曼体外诊断试剂直销和经销情况对比(单位:万元,%)
表:北京利德曼募资扩产诊断试剂项目
表:截止2011年7月北京利德曼申请产品海外注册进展
图:2008-2009年万泰生物经营情况
图:2008-2009年上海荣盛营业情况
图:2008年深圳匹基经营情况
表:深圳匹基检测试剂
图:2008-2009年奥普生物经营情况
表:奥普生物诊断试剂和诊断仪器
图:2008-2009年蓝十字经营情况
图:2008-2009年上海英伯肯医学生物技术有限公司经营情况
图:2008-2009年贝索生物经营情况
图:2008-2009年北方生物经营情况
图:2009年波生生物经营情况
Global Biopharmaceutical Market Scale, 2007-2012
Global Biopharmaceutical Revenue by Region, 2009
Market Size of IVD Industry Worldwide, 2008-2012
China’s Biopharmaceutical Sales and YoY Growth, 2005-Jan-May 2011
Relevant Polices of Diagnostic Reagent Industry in China
Product Structure of Diagnostic Reagent Industry in China, 2010
Structure of In Vitro Diagnostic Reagents in China, 2010
Market Size and Growth Rate of In Vitro Diagnostic Industry in China, 2007-2014
Market Size and Growth Rate of In Vitro Diagnostic Reagents in China, 2007-2014
Major Manufacturers of IVD Products in China
China’s Import Value, Import Volume and Average Price of Contrast Agent for X-Ray Inspection and Diagnostic Reagent for Patients, 2007-Jan.-Aug. 2011
China’s Accumulated Import Volume & Value and Proportion of Contrast Agent for X-Ray Inspection and Diagnostic Reagent for Patients by Country of Origin, Jan.-Aug. 2011
Distribution of China’s Imported Contrast Agent for X-Ray Inspection and Diagnostic Reagent for Patients by Destination, 2008- Jan- Aug.2011
China’s Export Value, Export Volume and Average Price of Contrast Agent for X-Ray Inspection and Diagnostic Reagent for Patients by Destination, 2007-Jan.-Aug. 2011
China’s Accumulated Export Volume & Value and Proportion of Contrast Agent for X-Ray Inspection and Diagnostic Reagent for Patients by Destination, Jan.-Aug. 2011
Export Origins Distribution of China’s Contrast Agent for X-Ray Inspection and Diagnostic Reagent for Patients, Jan.-Aug. 2011
China’s Biochemical Reagent Market Scale, 2007-2014
China’s Immune Reagent Market Scale, 2007-2014
Operating Revenue and Net Income of KHB, 2006- H1 2011
Operating Revenue of KHB by Product, 2007- H1 2011
Gross Margin of KHB by Product, 2008-H1 2011
Proportion of Operating Revenue of KHB by Region, 2008-H1 2011
Diagnostic Reagent Products of KHB
Operating Revenue and Gross Margin of KHB, 2011-2013E
Operating Revenue and Net Income of Da An Gene, 2006- H1 2011
Operating Revenue of Da An Gene by Product, 2007- H1 2011
Gross Margin of Da An Gene by Product, 2008-H1 2011
Proportion of Operating Revenue of Da An Gene by Region, 2008-H1 2011
R&D Input and Its Ratio to Operating Revenue of Da An Gene, 2008-2010
Operating Revenue and Gross Margin of Da An Gene, 2011-2013E
Operating Revenue and Net Income of Fosun Pharmaceutical (Group), 2006- H1 2011
Operating Revenue and Gross Margin of Fosun Pharmaceutical (Group), 2011-2013 E
Operation of Shanghai Fosun Long March Medical Science, 2008-2009
Operation of Shanghai Fosun Med-Tech Development, 2008-2009
Operating Revenue and Net Income of Biosino Bio-Technology & Science, 2006- H1 2011
Operating Revenue of Biosino Bio-Technology & Science by Product, 2008- H1 2011
Licenses and Patents for In-vitro Diagnostic Reagents of Biosino Bio-Technology& Science, 2010
Operating Revenue and Net Income of Beijing Kinghawk Pharmaceutical, 2008- H1 2011
Operating Revenue of Beijing Kinghawk Pharmaceutical (by Product), 2010- H1 2011
Operating Revenue of Beijing Kinghawk Pharmaceutical (by Region), 2010- H1 2011
Operating Revenue of Zhuhai Livzon Diagnostics, 2007- H1 2011
Certificate Acquired by Zhuhai Livzon Diagnostics, Jun.2010-Sep.2011
Operation of ACON Biotech, 2008-2009
Certificate Acquired by ACON Biotech for the Registration of Medical Devices, Mar. 2010 & May 2011
Capacity, Output and Sales Volume of Beijing Leadman’ s IVO Reagent, 2008-2011(Kiloliter )
Output and Sales Volume Breakdonw of Beijing Leadman’s IVO Reagent 2010-2011(Liter)
Sales Revenue and Its Proportion of Beijing Leadman’s IVO Reagent, 2008-2011(RMB mIn)
Sale Model of Beijing Leadman’s IVO Reagent, 2008-2011
New Projects of Beijing Leadman’s IVO Reagent by IPO
Progress of Overseas Product Registration of Beijing Leadman by Jul.2011
Operation of Beijing Wantai Biological Pharmacy, 2008-2009
Operation of Shanghai Rongsheng Biotech, 2008-2009
Operation of PG Biotech, 2008
Detection Reagent of PG Biotech
Operation of Shanghai Upper Bio-Tech Pharma, 2008-2009
Diagnostic Reagents and Diagnostic Instruments of Shanghai Upper Bio-Tech Pharma
Operation of Blue Cross Bio-Medical (Beijing), 2008-2009
Operation of Inverness Medical (Shanghai), 2008-2009
Operation of Baso Diagnostics, 2008-2009
Operation of Beijing North Institute of Biological Technology, 2008-2009
Operation of Boson Biotech, 2009
如果这份报告不能满足您的要求,我们还可以为您定制报告,请 留言说明您的详细需求。
|