|
|
|
报告导航:研究报告—
生命科学—生物科技
|
|
2014-2017年全球及中国干细胞产业研究报告 |
 |
字数:3.2万 |
页数:105 |
图表数:106 |
|
中文电子版:8000元 |
中文纸版:4000元 |
中文(电子+纸)版:8500元 |
|
英文电子版:2250美元 |
英文纸版:2400美元 |
英文(电子+纸)版:2550美元 |
|
编号:PQ012
|
发布日期:2014-09 |
附件:下载 |
 |
|
|
干细胞是一种具有无限增殖和分化潜能的细胞,具有再生各种组织器官的功能。干细胞治疗可应用于心血管系统疾病、血液系统疾病中的白血病、神经系统疾病、肝肾等实质器官损伤或病变等。
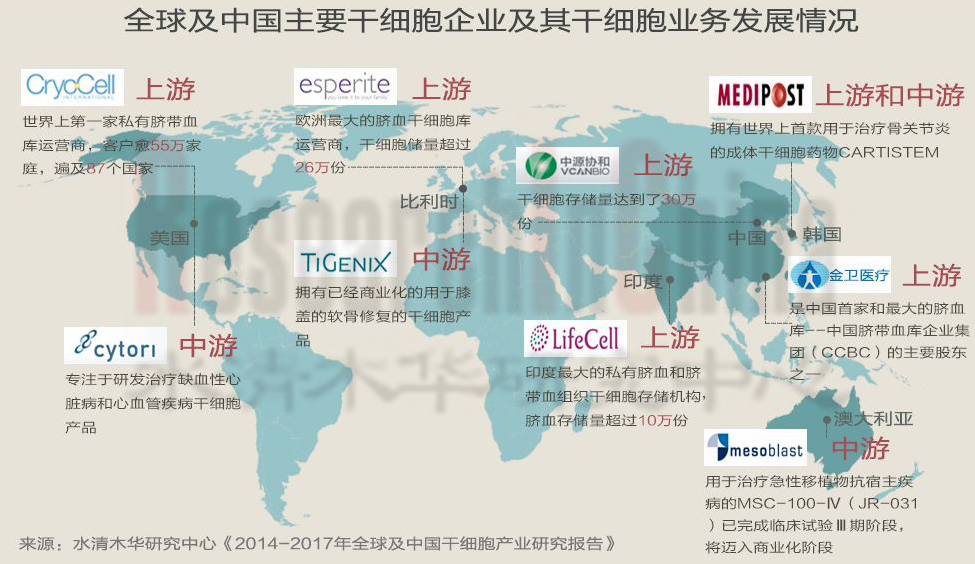
目前,脐带血库是干细胞上游乃至整个产业链中发展最快且相对成熟的市场。2005年,全球有23家脐带血库,2013年超过了480家。全球的脐血干细胞存储企业大致可以分为两类:一是采取全球化经营模式企业,如Cryo-Cell International、Esperite;另一类以区域性经营为主企业,如中源协和、金卫医疗和LifeCell international。不过,目前主营脐血库业务企业的单体规模并不大,只有个别企业客户数超过50万。
而以干细胞技术及产品研发为主的中游产业正处于发展初期,主要集中于欧美以及韩国等少数国家。目前大多数处于该产业链环节的企业由于研发支出巨大,基本处于连年亏损状态。不过由于干细胞治疗领域巨大的市场潜力,再加上政策大力扶持(如资金补贴)以及重要合作伙伴资金支持,鲜有企业从中退出。
截至目前,全球获批上市的干细胞产品共有9款,其中3款属于干细胞药物,均由韩国企业所研发,如MEDIPOST用于治疗骨关节炎的成体干细胞药物CARTISTEM和用于治疗儿童急性移植物抗宿主病(GVHD)干细胞产品—Prochymal(2013年10月,MEDIPOST通过收购Osiris Therapeutics的Therapeutics业务,获得该产品)。
与此同时,传统制药巨头如诺华(Novartis)等正着手通过兼并收购策略,快速进入该领域。2014年8月19日,诺华公司同Gamida Cell(一家致力于干细胞技术研发并将其应用到白血病患者干细胞移植的企业)达成收购协议,即诺华出资3500万美元收购后者15%股权,并拥有2年内以1.65亿美元收购后者剩余股权的选择权;2013年 9 月,诺华公司还与 Regenerex 达成合作,共同开发后者的造血干细胞平台 FCRx。
《2014-2017年全球及中国干细胞产业研究报告》主要内容如下:
 干细胞分类、应用、产业链定义等; 干细胞分类、应用、产业链定义等;
 全球干细胞主要企业、政策、上中下游发展情况及前景等; 全球干细胞主要企业、政策、上中下游发展情况及前景等;
 中国干细胞产业政策、上中下游发展情况等; 中国干细胞产业政策、上中下游发展情况等;
 全球上游6家、中下游19家企业的经营情况、干细胞业务等。 全球上游6家、中下游19家企业的经营情况、干细胞业务等。
Stem cells are undifferentiated biological cells that can differentiate into specialized cells and can divide (through mitosis) to produce more stem cells. Stem cell therapy can be applied to treatment of cardiovascular diseases, leukemia (a kind of hematological system disease), nervous system diseases, damage or lesion of liver, kidney and other parenchymal organs, etc..
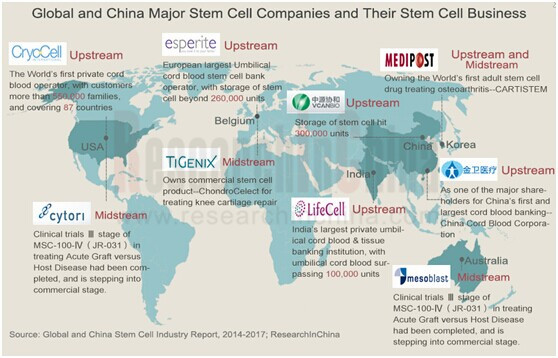
Currently, cord blood bank is the fastest-growing and relatively mature market amid stem cell upstream sectors and even the whole industry chain. In 2005, there were 23 cord blood banks worldwide and in 2013 the figure exceeded 480. Global cord blood stem cell (CBSC) storage companies can be roughly divided into two categories: the ones running in a globalized business model, such as Cryo-Cell International and Esperite (formerly known as Cryo-Save Group), and the others giving priority to regional operation e.g. Zhongyuan Union Stem Cell Bioengineering (VCANBIO), Golden Meditech and LifeCell International. However, the companies mainly engaged in cord blood bank business are currently small in scale, only a few with more than 500,000 clients.
The stem cell technology and product research-oriented midstream sector is in its infancy, mostly concentrated in few countries like Europe, America and South Korea. At present, most companies in the industry chain are basically in the red for years running due to huge R&D costs. Nevertheless, attracted by the tremendous market potential in the area of stem cell therapy and enjoying the great encouragement from government policies (e.g. capital subsidy) and the capital support of significant cooperative partners, very few companies have dropped out.
Up to now, altogether 9 sorts of stem cell products have been approved worldwide, 3 of which are in the category of stem cell drugs developed by S. Korean companies, such as MEDIPOST’s adult stem cell drug CARTISTEM for osteoarthritis treatment and the stem cell product Prochymal (MEDIPOST obtained the product via acquiring the Therapeutics business of Osiris Therapeutics) direct at treating children suffering acute graft-versus-host disease (GVHD).
In the meantime, traditional pharmaceutical giants like Novartis are setting about quickly accessing the field through mergers and acquisitions. On Aug. 19, 2014, Novartis reached an acquisition agreement with Gamida Cell (a corporate dedicated to stem cell technology R&D and its application in stem cell transplantation for leukemia patients), which specified that Novartis spend USD35 million in acquiring 15% equity in the latter and win the option to take over the remaining equity in two years with USD165 million; in Sep. 2013, Novartis also entered a cooperation with Regenerex to jointly develop the hematopoietic stem cell platform FCRx of the latter.
Global and China Stem Cell Industry Report, 2014-2017 highlights the followings:
 Classification, application, industry chain definition, etc. of stem cells; Classification, application, industry chain definition, etc. of stem cells;
 Major enterprises, policies, upstream/midstream/downstream development and prospects, etc. of global stem cell industry; Major enterprises, policies, upstream/midstream/downstream development and prospects, etc. of global stem cell industry;
 Policies, upstream/midstream/downstream development, etc. of China stem cell industry; Policies, upstream/midstream/downstream development, etc. of China stem cell industry;
 Operation, stem cell business, etc. of 6 upstream companies and 18 midstream/downstream companies worldwide. Operation, stem cell business, etc. of 6 upstream companies and 18 midstream/downstream companies worldwide.
第一章 干细胞行业概况
1.1定义
1.2分类与应用
1.3 产业链
1.4 发展历程
第二章 全球干细胞行业产业链研究
2.1 主要企业产业链布局
2.2 政策概况
2.3发展状况
2.4 上游
2.5 下游
2.6前景
第三章 中国干细胞产业链研究
3.1 发展简介
3.2 政策环境
3.3 上游
3.4 中游
3.5 下游
第四章 上游企业
4.1 Cryo-Cell International
4.1.1 公司简介
4.1.2 经营情况
4.1.3 脐带血业务
4.2 Esperite
4.2.1 公司简介
4.2.2 经营情况
4.2.3 营收构成
4.2.4 干细胞业务
4.3 中源协和
4.3.1 公司简介
4.3.2 经营情况
4.3.3 营收构成
4.3.4 毛利率
4.3.5 研发与投资
4.3.6 干细胞业务
4.3.7发展预测
4.4 金卫医疗
4.4.1 公司简介
4.4.2 经营情况
4.4.3营收构成
4.4.4 利润
4.4.5 脐带血业务
4.4.6 发展预测
4.5 LifeCell international
4.5.1 公司简介
4.5.2 脐带血业务
4.6 Cryosite
4.6.1 公司简介
4.6.2 经营情况
4.6.3 营收构成
4.6.4 干细胞业务
第五章 中下游企业
5.1 NeoStem
5.1.1 公司简介
5.1.2 经营情况
5.1.3 营收构成
5.1.4 研发
5.1.5 干细胞业务
5.2 Mesoblast
5.2.1 公司简介
5.2.2 经营情况
5.2.3 研发与投资
5.2.4 干细胞业务
5.3 MEDIPOST
5.3.1 公司简介
5.3.2 经营情况
5.3.3 干细胞业务
5.4北科生物
5.4.1 公司简介
5.4.2 干细胞业务
5.5 Osiris Therapeutics
5.5.1 公司简介
5.5.2 经营情况
5.5.3 研发
5.6 Orthofix International
5. 6.1 公司简介
5.6.2 经营情况
5.6.3 营收构成
5.6.4 研发
5.6.5 干细胞业务
5.7 Cytori Therapeutics
5.7.1 公司简介
5.7.2 经营情况
5.7.3 研发
5.8 Athersys
5.8.1 公司简介
5.8.2 经营情况
5.8.3 研发
5.9 Pluristem Therapeutics
5.9.1 公司简介
5.9.2 研发
5.9.3 最新动态
5.10 StemCells
5.10.1 公司简介
5.10.2 经营情况
5.10.3 研发
5.11 Advanced Cell Technology
5.11.1 公司简介
5.11.2 经营情况
5.11.3 研发
5.12 Celgene
5.12.1 公司简介
5.12.2 经营情况
5.12.3 研发
5.13 Vitro Biopharma
5.13.1 公司简介
5.13.2 经营情况
5.13.3 研发
5.13.4 干细胞业务
5.14 Reneuron
5.14.1 公司简介
5.14.2 经营情况
5.14.3 研发
5.15 Tigenix
5.15.1 公司简介
5.15.2 经营情况
5.15.3 研发
5.16 西比曼生物科技
5.16.1 公司简介
5.16.2 经营情况
5.16.3 研发
5.16.4干细胞业务
5.16.5 在华业务
5.17 Opexa Therapeutics
5.18 中科生物
5.19 赛业生物科技
1. Overview of Stem Cell Industry
1.1 Definition
1.2 Classification & Application
1.3 Industry Chain
1.4 Development Course
2. Global Stem Cell Industry Chain
2.1 Industry Chain Layout of Major Enterprises
2.2 Policy
2.3 Development
2.4 Upstream
2.5 Downstream
2.6 Prospect
3. China Stem Cell Industry Chain
3.1 Development Introduction
3.2 Policy Environment
3.3 Upstream
3.4 Midstream
3.5 Downstream
4. Upstream Enterprises
4.1 Cryo-Cell International
4.1.1 Profile
4.1.2 Operation
4.1.3 Cord Blood Business
4.2 Esperite
4.2.1 Profile
4.2.2 Operation
4.2.3 Revenue Structure
4.2.4 Stem Cell Business
4.3 Zhongyuan Union Stem Cell Bioengineering
4.3.1 Profile
4.3.2 Operation
4.3.3 Revenue Structure
4.3.4 Gross Margin
4.3.5 R&D and Investment
4.3.6 Stem Cell Business
4.3.7 Development Forecast
4.4 Golden Meditech
4.4.1 Profile
4.4.2 Operation
4.4.3 Revenue Structure
4.4.4 Profit
4.4.5 Cord Blood Business
4.4.6 Development Forecast
4.5 LifeCell International
4.5.1 Profile
4.5.2 Cord Blood Business
4.6 Cryosite
4.6.1 Profile
4.6.2 Operation
4.6.3 Revenue Structure
4.6.4 Stem Cell Business
5. Midstream and Downstream Enterprises
5.1 NeoStem
5.1.1 Profile
5.1.2 Operation
5.1.3 Revenue Structure
5.1.4 R&D
5.1.5 Stem Cell Business
5.2 Mesoblast
5.2.1 Profile
5.2.2 Operation
5.2.3 R&D and Investment
5.2.4 Stem Cell Business
5.3 MEDIPOST
5.3.1 Profile
5.3.2 Operation
5.3.3 Stem Cell Business
5.4 Beike Biotechnology
5.4.1 Profile
5.4.2 Stem Cell Business
5.5 Osiris Therapeutics
5.5.1 Profile
5.5.2 Operation
5.5.3 R&D
5.6 Orthofix International
5.6.1 Profile
5.6.2 Operation
5.6.3 Revenue Structure
5.6.4 R&D
5.6.5 Stem Cell Business
5.7 Cytori Therapeutics
5.7.1 Profile
5.7.2 Operation
5.7.3 R&D
5.8 Athersys
5.8.1 Profile
5.8.2 Operation
5.8.3 R&D
5.9 Pluristem Therapeutics
5.9.1 Profile
5.9.2 R&D
5.9.3 Recent News
5.10 StemCells
5.10.1 Profile
5.10.2 Operation
5.10.3 R&D
5.11 Advanced Cell Technology
5.11.1 Profile
5.11.2 Operation
5.11.3 R&D
5.12 Celgene
5.12.1 Profile
5.12.2 Operation
5.12.3 R&D
5.13 Vitro Biopharma
5.13.1 Profile
5.13.2 Operation
5.13.3 R&D
5.13.4 Stem Cell Business
5.14 Reneuron
5.14.1 Profile
5.14.2 Operation
5.14.3 R&D
5.15 Tigenix
5.15.1 Profile
5.15.2 Operation
5.15.3 R&D
5.16 Cellular Biomedicine Group, Inc. (CBMG)
5.16.1 Profile
5.16.2 Operation
5.16.3 R&D
5.16.4 Stem Cell Business
5.16.5 Business in China
5.17 Opexa Therapeutics
5.18 ZhongKeBiopharm
5.19 Cyagen Biosciences
表:干细胞分类
表:干细胞治疗领域概况(按疾病类型)
表:干细胞治疗的优缺点分析
表:成体干细胞来源及应用(分类型)
图:干细胞产业链
表:全球主要干细胞企业产业链分布
表:全球已获得上市许可干细胞产品及其临床应用
表:截至2014年上半年全球干细胞治疗主要在研产品、治疗领域及其临床试验阶段
图:2007-2013年全球干细胞市场规模及同比增长
图:2013年全球人体干细胞临床试验及治疗应用构成
图:2013年全球干细胞技术临床应用(分疾病类型)
表:截至2014年上半年全球主要国家脐带血库分布
表:全球主要国家脐带血应用情况
图:2012年全球前10大致死原因排名(按死亡人数)
图:2012年全球前10大致死原因对比(按收入分组)
图:2012年全球低收入国家前10大致死原因排名
图:2012年全球中低收入国家前10大致死原因排名
图:2012年全球中高收入国家前10大致死原因排名
图:2012年全球高收入国家前10大致死原因排名
图:全球干细胞应用前景
图:2014-2017年全球干细胞市场规模及同比增长预测
表:中国部分已获得CFDA批准的干细胞临床试验药物
表:1999-2014年中国干细胞产业政策
表:中国干细胞产业上游主要企业及其干细胞业务
表:中国干细胞产业中游主要企业及其干细胞业务
图:2007-2017年中国干细胞医疗市场规模及同比增长
图:2010-2014财年Cryo-Cell International营业收入、净利润及毛利率
图:2010-2014财年Cryo-Cell International总资产、总负债及资产负债率
图:2012-2014财年Cryo-Cell International脐带血业务收入及其营收占比
图:2009-2014年Esperite营业收入、净利润及营业利润率
图:2009-2014年Esperite(分业务)营业收入
图:2012和2013年Esperite(分地区)营业收入构成对比
表:2009-2014年Esperite干细胞存储量
图:2009-2014年Esperite干细胞存储收入及其EBITA
表:截至2014年上半年中源协和主要子公司
图:2009-2014年中源协和营业收入和净利润
表:2012-2014年中源协和主要子公司营业收入、净利润
图:2011-2014年中源协和(分业务)营业收入
表:2010-2014年中源协和(分地区)营业收入
表:2011-2014年中源协和(分业务)营业收入
图:2009-2014年中源协和研发支出及其营收占比
表:2009-2013年中源协和干细胞(分类别)存储业务
图:2014-2017年中源协和营业收入和净利润预测
图:2009-2013财年金卫医疗营业收入、净利润
图:2009-2013财年金卫医疗(分业务)营业收入
表:2010-2013财年金卫医疗(分业务)利润
图:2011-2014财年金卫医疗脐带血库营业收入及其占比
图:2011-2014财年金卫医疗脐带血库毛利润及毛利率
表: 金卫医疗脐带血存储业务发展历程
图:2014-2017财年金卫医疗营业收入和净利润预测
表:LifeCell international发展历程
图:2011-2014财年Cryosite营业收入和净利润
表:2011-2014财年 Cryosite(分业务)营业收入及EBITDA
表:2009-2014年 NeoStem并购历程
图:2009-2014年NeoStem营业收入和净利润
图:2012-2014年NeoStem(分业务)营业收入
图:2009-2014年NeoStem研发支出及其营收占比
表:2014年NeoStem临床试验项目
图:2009-2014财年Mesoblast营业收入和净利润
图:2009-2014财年Mesoblast研发支出及其营收占比
表:截至2013年底Mesoblast并购历程
表:截至2014年6月底Mesoblast干细胞产品研发进程
图:2011-2013财年MEDIPOST营业收入、净利润及资产负债率
表:MEDIPOST成体干细胞药物及治疗领域
表:北科生物发展历程
表:Osiris Therapeutics旗下干细胞品牌及其治疗领域
图:2011-2014年Osiris Therapeutics营业收入、净利润及毛利率
图:2011-2014年Osiris Therapeutics研发支出及其营收占比
图:2009-2014年Orthofix International营业收入、净利润及毛利率
图:2011-2014年Orthofix International(分部门)营业收入
图:2011-2013年Orthofix International(分地区)营业收入
图:2009-2013年Orthofix International研发支出及其营收占比
表:2011-2013年Orthofix International干细胞业务收入
图:2009-2014年Cytori Therapeutics营业收入、净利润及毛利率
图:2010-2014年Cytori Therapeutics(分地区)营业收入
图:2009-2014年Cytori Therapeutics研发支出及其营收占比
表:截至2014年上半年Cytori Therapeutics干细胞产品研发、治疗领域及进程
图:2009-2014年Athersys营业收入和净利润
图:2009-2014年Athersys(按来源)营业收入
图:2009-2014年Athersys研发支出及其营收占比
表:截至2014年上半年Athersys干细胞产品研发、治疗领域及进程
表:Athersys重要合作伙伴及合作领域
表:截至2014年3月底Pluristem Therapeutics干细胞产品研发、治疗领域及进程
图:2009-2014年StemCells营业收入、净利润及毛利率
图:2009-2014年StemCells研发支出及其营收占比
表: 截至2014年上半年StemCells干细胞产品研发、治疗领域及进程
图:2009-2014年ACT营业收入、净利润及毛利率
图:2009-2014年ACT研发支出及其营收占比
表:截至2013年底ACT干细胞产品研发、治疗领域及进程
图:2009-2014年Celgene营业收入、净利润及营业利润率
图:2009-2013年Celgene细胞治疗研发支出及其营收占比
表:截至2014年上半年Celgene干细胞产品研发、治疗领域及进程
图:2009-2014财年Vitro Biopharma营业收入、净利润及毛利率
图:2011-2014年Vitro Biopharmas研发支出及其营收占比
表:Vitro Biopharma旗下干细胞品牌及其应用领域
图:2009-2013财年Celgene营业收入和净利润
图:2009-2013年Reneuron研发支出及其营收占比
表:截至2013年底Reneuron干细胞产品研发、治疗领域及进程
图:2009-2014年 Tigenix营业收入和净利润
图:2011-2013年Tigenix(分地区)销售收入
图:2009-2014年Tigenix研发支出及其营收占比
表:截至2014年上半年Tigenix干细胞产品研发、治疗领域及进程
表:西比曼生物科技发展历程
图:2009-2014年西比曼生物科技营业收入、净利润及营业利润率
图:2012-2014年西比曼生物科技研发支出及其营收占比
表:截至2014年上半年西比曼生物科技干细胞产品研发、治疗领域及进程
Classification of Stem Cell
Overview of Stem Cell Therapy Area (by Disease Type)
Advantages and Disadvantages of Stem Cell Therapy
Source and Application of Adult Stem Cells (by Type)
Stem Cell Industry Chain
Industry Chain Distribution of Major Global Stem Cell Enterprises
Global Authorized Stem Cell Products and Their Clinical Application
Major Products under Development, Therapeutic Area and Clinical Trial Stage of Global Stem Cell Therapy as of 2014H1
Global Stem Cell Market Size and YoY Growth, 2007-2013
Global Human Stem Cell Clinical Trials and Treatment Application Structure, 2013
Clinical Application of Global Stem Cell Technology (by Disease Type), 2013
Cord Blood Bank Distribution in Major Countries as of 2014H1
Cord Blood Application in Major Countries
Global Top 10 Death Causes (by Death Toll), 2012
Global Top 10 Death Causes (by Income Group), 2012
Global Top 10 Death Causes in Low-income Countries, 2012
Global Top 10 Death Causes in Low- and Middle-income Countries, 2012
Global Top 10 Death Causes in Middle- and High-income Countries, 2012
Global Top 10 Death Causes in High-income Countries, 2012
Global Stem Cell Application Prospect
Global Stem Cell Market Size and YoY Growth, 2014-2017E
Some Chinese Stem Cell Clinical Trial Drugs Approved by CFDA
China Stem Cell Industry Policies, 1999-2014
Major Enterprises and Their Stem Cell Business in China Upstream Stem Cell Industry
Major Enterprises and Their Stem Cell Business in China Midstream Stem Cell Industry
China Stem Cell Medical Market Size and YoY Growth, 2007-2017E
Revenue, Net Income and Gross Margin of Cryo-Cell International, FY2010-FY2014
Total Assets, Total Liabilities and Asset-liability Ratio of Cryo-Cell International, FY2010-FY2014
Cord Blood Business Revenue and % of Total Revenue of Cryo-Cell International, FY2012-FY2014
Revenue, Net Income and Operating Margin of Esperite, 2009-2014
Revenue Breakdown of Esperite (by Business), 2009-2014
Revenue Structure of Esperite (by Region), 2012VS2013
Stem Cell Storage of Esperite, 2009-2014
Stem Cell Storage Revenue and EBITA of Esperite, 2009-2014
Main Subsidiaries of Zhongyuan Union Stem Cell Bioengineering as of 2014H1
Revenue and Net Income of Zhongyuan Union Stem Cell Bioengineering, 2009-2014
Revenue and Net Income of Zhongyuan Union Stem Cell Bioengineering’s Major Subsidiaries, 2012-2014
Revenue Breakdown of Zhongyuan Union Stem Cell Bioengineering (by Business), 2011-2014
Revenue Breakdown of Zhongyuan Union Stem Cell Bioengineering (by Region), 2010-2014
Gross Margin of Zhongyuan Union Stem Cell Bioengineering (by Business), 2011-2014
R&D Costs and % of Total Revenue of Zhongyuan Union Stem Cell Bioengineering, 2009-2014
Stem Cell Storage Business of Zhongyuan Union Stem Cell Bioengineering (by Type), 2009-2013
Revenue and Net Income of Zhongyuan Union Stem Cell Bioengineering, 2014-2017E
Revenue and Net Income of Golden Meditech, FY2009-FY2013
Revenue Breakdown of Golden Meditech (by Business), FY2009-FY2013
Profit Breakdown of Golden Meditech (by Business), FY2010-FY2013
Cord Blood Bank Revenue and % of Total Revenue of Golden Meditech, FY2011-FY2014
Cord Blood Bank Gross Profit and Gross Margin of Golden Meditech, FY2011-FY2014
Development Course of Golden Meditech’s Cord Blood Storage Business
Revenue and Net Income of Golden Meditech, FY2014-FY2017E
Development Course of LifeCell International
Revenue and Net Income of Cryosite, FY2011-FY2014
Revenue and EBITDA of Cryosite (by Business), FY2011-FY2014
M&A Course of NeoStem, 2009-2014
Revenue and Net Income of NeoStem, 2009-2014
Revenue Breakdown of NeoStem (by Business), 2012-2014
R&D Costs and % of Total Revenue of NeoStem, 2009-2014
Clinical Trial Projects of NeoStem, 2014
Revenue and Net Income of Mesoblast, FY2009-FY2014
R&D Costs and % of Total Revenue of Mesoblast, FY2009-FY2014
M&A Course of Mesoblast by the end of 2013
Stem Cell Product R&D Progress of Mesoblast by the end of June 2014
Revenue, Net Income and Asset-liability Ratio of MEDIPOST, FY2011-FY2013
Adult Stem Cell Drugs and Therapeutic Area of MEDIPOST
Development Course of Beike Biotechnology
Stem Cell Brands and Therapeutic Area of Osiris Therapeutics
Revenue, Net Income and Gross Margin of Osiris Therapeutics, 2011-2014
R&D Costs and % of Total Revenue of Osiris Therapeutics, 2011-2014
Revenue, Net Income and Gross Margin of Orthofix International, 2009-2014
Revenue Breakdown of Orthofix International (by Department), 2011-2014
Revenue Breakdown of Orthofix International (by Region), 2011-2013
R&D Costs and % of Total Revenue of Orthofix International, 2009-2013
Stem Cell Business Revenue of Orthofix International, 2011-2013
Revenue, Net Income and Gross Margin of Cytori Therapeutics, 2009-2014
Revenue Breakdown of Cytori Therapeutics (by Region), 2010-2014
R&D Costs and % of Total Revenue of Cytori Therapeutics, 2009-2014
Stem Cell Products R&D, Therapeutic Area and Progress of Cytori Therapeutics as of 2014H1
Revenue and Net Income of Athersys, 2009-2014
Revenue Breakdown of Athersys (by Source), 2009-2014
R&D Costs and % of Total Revenue of Athersys, 2009-2014
Stem Cell Products R&D, Therapeutic Area and Progress of Athersys as of 2014H1
Significant Cooperative Partners and Areas of Athersys
Stem Cell Products R&D, Therapeutic Area and Progress of Pluristem Therapeutics by the end of Mar. 2014
Revenue, Net Income and Gross Margin of StemCells, 2009-2014
R&D Costs and % of Total Revenue of StemCells, 2009-2014
Stem Cell Products R&D, Therapeutic Area and Progress of StemCells as of 2014H1
Revenue, Net Income and Gross Margin of ACT, 2009-2014
R&D Costs and % of Total Revenue of ACT, 2009-2014
Stem Cell Products R&D, Therapeutic Area and Progress of ACT by the end of 2013
Revenue, Net Income and Operating Margin of Celgene, 2009-2014
Cellular Therapy R&D Costs and % of Total Revenue of Celgene, 2009-2013
Stem Cell Products R&D, Therapeutic Area and Progress of Celgene as of 2014H1
Revenue, Net Income and Gross Margin of Vitro Biopharma, FY2009-FY2014
R&D Costs and % of Total Revenue of Vitro Biopharmas, 2011-2014
Stem Cell Brands and Application Fields of Vitro Biopharma
Revenue and Net Income of Celgene, FY2009-FY2013
R&D Costs and % of Total Revenue of Reneuron, 2009-2013
Stem Cell Products R&D, Therapeutic Area and Progress of Reneuron by the end of 2013
Revenue and Net Income of Tigenix, 2009-2014
Sales of Tigenix (by Region), 2011-2013
R&D Costs and % of Total Revenue of Tigenix, 2009-2014
Stem Cell Products R&D, Therapeutic Area and Progress of Tigenix as of 2014H1
Development Course of CBMG
Revenue, Net Income and Operating Margin of CBMG,2009-2014
R&D Costs and % of Total Revenue of CBMG, 2012-2014
Stem Cell Products R&D,Therapeutic Area and Progress of CBMG as of 2014H1
如果这份报告不能满足您的要求,我们还可以为您定制报告,请 留言说明您的详细需求。
|