|
|
|
报告导航:研究报告—
生命科学—制药医疗
|
|
2016-2021年中国干扰素行业研究报告 |
 |
字数:2.9万 |
页数:83 |
图表数:104 |
|
中文电子版:11000元 |
中文纸版:5500元 |
中文(电子+纸)版:11500元 |
|
英文电子版:2400美元 |
英文纸版:2600美元 |
英文(电子+纸)版:2700美元 |
|
编号:ZLC-047
|
发布日期:2017-04 |
附件:下载 |
 |
|
|
干扰素主要用于乙肝、丙肝、多发性硬化症、尖锐湿疣等病症的治疗,其中IFN-α主要用于乙肝和丙肝的治疗,IFN-β则是MS的主流药物。MS在欧美的发病率较高,亚洲较低,因此国外主要以IFN-β为主,中国临床应用主要以IFN-α为主。
中国是肝病大国,近年来对干扰素的需求保持在40亿以上,其中乙肝市场占整个干扰素市场的60%以上。但是2016年在专利药到期以及核苷类药物替代等因素的冲击下,干扰素乙肝市场迅速萎缩,导致2016年中国样本医院干扰素销售额大幅下降至3.61亿元,同比下降50.4%,估计2016年中国干扰素行业市场规模约22.2亿元。
预计未来几年,中国干扰素市场将难以扭转下滑局面,除了受乙肝市场走低影响外,受DAAs药物的陆续上市影响,丙肝市场也将面临危机。目前海外丙肝用干扰素市场已受DAAs药物影响大幅下滑,国内虽未有DAAs药物上市,但也有30多家企业申报DAAs药物,预计近2年将有DAAs药物上市,届时将再次冲击国内干扰素市场。
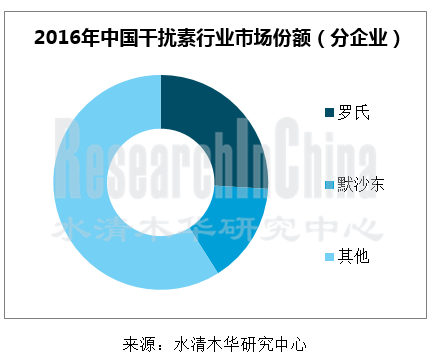
近年来,中国干扰素市场主要被罗氏和默沙东所占据,二者占据了60%以上的市场份额,而以安科生物、凯因科技、三元基因为代表的本土企业各自市场份额占比不到5%。不过2016年,随着乙肝市场的萎缩,罗氏和默沙东的市场份额大幅下滑,合计占比降至40%左右。
干扰素主要分为普通干扰素和长效干扰素。与普通干扰素每周注射三次相比,长效干扰素减少到每周一次,患者的用药依从性也可以得到明显改善,同时副作用更小。但中国长效干扰素市场一直以来被罗氏和默沙东所垄断,价格高昂。
2016年10月13日,由特宝生物研发的中国首个具有自主知识产权的长效干扰素获批上市,打破了国外企业的垄断。同时安科生物、三元基因、正大天晴等在内的企业的长效干扰素产品正处于研发之中。未来,中国长效干扰素市场竞争将更为激烈,罗氏、默沙东的市场份额将被进一步挤压。
《2016-2021年中国干扰素行业研究报告》主要包括以下内容:
 中国干扰素行业发展概况分析,包括发展历程、发展现状、市场规模、市场结构及竞争格局分析; 中国干扰素行业发展概况分析,包括发展历程、发展现状、市场规模、市场结构及竞争格局分析;
 中国干扰素行业细分市场分析,分别对普通干扰素和长效干扰素的市场规模、竞争格局、价格对比进行了深入分析; 中国干扰素行业细分市场分析,分别对普通干扰素和长效干扰素的市场规模、竞争格局、价格对比进行了深入分析;
 中国干扰素行业下游市场分析,分别对乙肝、丙肝、儿科市场的发展现状、发展趋势进行分析; 中国干扰素行业下游市场分析,分别对乙肝、丙肝、儿科市场的发展现状、发展趋势进行分析;
 对国内17家、国外2家干扰素企业的经营情况、干扰素业务进行了深入分析; 对国内17家、国外2家干扰素企业的经营情况、干扰素业务进行了深入分析;
 总结与预测。 总结与预测。
Interferon is mainly used for the treatment of hepatitis B, hepatitis C, multiple sclerosis, condyloma acuminatum and other diseases. Wherein, IFN-α is mainly suitable for curing hepatitis B and hepatitis C, while IFN-β is the mainstream medicine for MS which sees a higher incidence in Europe and America than in Asia, so foreign countries usually adopt IFN-β whereas clinical applications in China center on IFN-α.
China where liver diseases prevail has maintained the demand for interferon worth more than RMB4 billion in recent years, of which hepatitis B-use interferon accounts for over 60% of the entire interferon market. However, the hepatitis B-use interferon market rapidly shrank in 2016 under the impact of the expired patented drugs, the substitution of nucleoside drugs and other factors, resulting in the sharp year-on-year decline of 50.4% in the interferon revenue (RMB361 million) of Chinese sample hospitals in 2016; it was estimated that Chinese interferon market size hit about RMB2.22 billion in the whole 2016.
It is expected that Chinese interferon market will be hard to turn the declining situation around in the next few years. Both of the sluggish hepatitis B-use interferon market and the launch of DAAs drugs will make the hepatitis C-use interferon market suffer a crisis. At present, the overseas hepatitis C-use interferon market has been significantly affected by DAAs drugs and slumped. Although DAAs drugs are not available in China, more than 30 companies have filed for DAAs drugs and DAAs drugs are expected to be revealed in this country within 2 years, which will impact the domestic interferon market once again then.
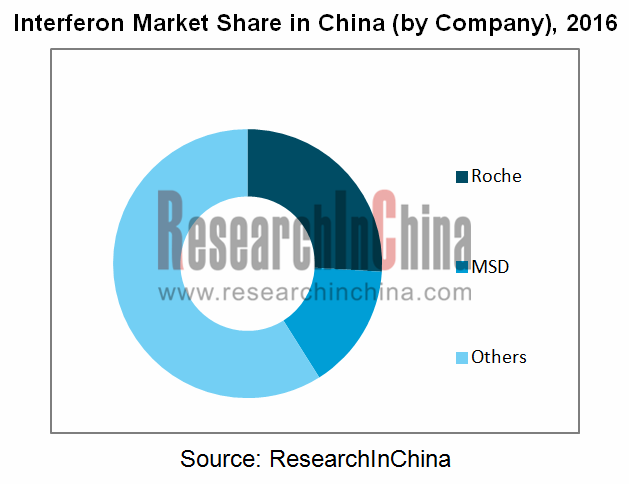
In recent years, Chinese interferon market is mainly occupied by Roche and MSD, which seize more than 60% market share together, while local Chinese peers represented by Anke Biotechnology, Kawin Technology and Tri-Prime Gene grasp no more than 5% apiece. But in 2016, the contracted hepatitis B-use interferon market dramatically dragged down the combined market share of Roche and MSD to about 40%.
Interferon is mainly divided into ordinary interferon and long-acting interferon. Compared with ordinary interferon which should be injected three times a week, long-acting interferon is injected only once a week, which significantly reduces the patients' medication compliance with less side effect. Yet, Chinese long-acting interferon market has been monopolized by Roche and MSD which offer high prices.
On October 13, 2016, China's first long-acting interferon developed by Amoytop Biotech with independent intellectual property rights obtained the approval for the launch, breaking the monopoly of foreign enterprises. Moreover, Anke Biotechnology, Tri-Prime Gene, Chia Tai Tianqing and other companies are developing long-acting interferon. In the future, the competition in Chinese long-acting interferon market will prick up, which will further squeeze the market share of Roche and MSD.
The report mainly covers the following:
 Overview of China interferon industry, including development process, status quo, market size, market structure and competitive landscape; Overview of China interferon industry, including development process, status quo, market size, market structure and competitive landscape;
 Analysis on China interferon market segments, especially in-depth analysis on market size, competitive landscape and prices of ordinary interferon and long-acting interferon; Analysis on China interferon market segments, especially in-depth analysis on market size, competitive landscape and prices of ordinary interferon and long-acting interferon;
 Analysis on the downstream market of China interferon industry, including status quo and development trend of hepatitis B, hepatitis C and pediatric markets; Analysis on the downstream market of China interferon industry, including status quo and development trend of hepatitis B, hepatitis C and pediatric markets;
 Operation and interferon business of 17 domestic and 2 foreign interferon enterprises; Operation and interferon business of 17 domestic and 2 foreign interferon enterprises;
 Summary and forecast. Summary and forecast.
第一章 概述
1.1 定义
1.2 分类
第二章 中国干扰素行业发展概况
2.1 发展历程
2.2 发展现状
2.3 市场规模
2.4 市场结构
2.5 竞争格局
第三章 中国干扰素行业细分市场分析
3.1 普通干扰素
3.1.1 简介
3.1.2 市场规模
3.1.3 竞争格局
3.1.4 价格对比
3.2 长效干扰素
3.2.1 简介
3.2.2 市场规模
3.2.3 竞争格局
3.2.4 价格对比
第四章 中国干扰素行业下游市场分析
4.1 乙肝
4.1.1 简介
4.1.2 治疗方式
4.1.3 干扰素乙肝市场萎缩
4.1.4 市场规模
4.2 丙肝
4.2.1 简介
4.2.2 治疗方式
4.2.3 DAAs药物上市冲击中国干扰素丙肝市场
4.3 儿科
4.4 其它
第五章 国内主要企业
5.1 安科生物
5.1.1 公司简介
5.1.2 经营情况
5.1.3 营收构成
5.1.4 研发与投资
5.1.5 干扰素业务
5.1.6 预测与展望
5.2 三元基因
5.2.1 公司简介
5.2.2 经营情况
5.2.3 研发情况
5.2.4 主要客户
5.2.5 干扰素业务
5.2.6 预测与展望
5.3 凯因科技
5.3.1 公司简介
5.3.2 经营情况
5.3.3 营收构成
5.3.4 研发与投资
5.3.5 主要客户
5.3.6 干扰素业务
5.3.7 预测与展望
5.4 特宝生物
5.4.1 公司简介
5.4.2 经营情况
5.4.3 干扰素业务
5.5 深圳科兴生物
5.5.1 公司简介
5.5.2 干扰素业务
5.6 未名医药
5.6.1 公司简介
5.6.2 干扰素业务
5.7 三生制药
5.7.1 公司简介
5.7.2 经营情况
5.7.3 干扰素业务
5.8 哈药生物
5.8.1 公司简介
5.8.2 经营情况
5.8.3 干扰素业务
5.9 长春生物制品研究所有限责任公司
5.9.1 公司简介
5.9.2 干扰素业务
5.10 浙江北生药业汉生制药有限公司
5.10.1 公司简介
5.10.2 干扰素业务
5.11 其它
5.11.1 海南欣明达生物制药有限公司
5.11.2 北京远策药业有限责任公司
5.11.3 黑龙江庆丰源生物工程技术有限责任公司
5.11.4 辽宁卫星生物制品研究所
5.11.5 上海凯茂生物医药有限公司
5.11.6 长春海伯尔生物技术有限责任公司
5.11.7 上海华新生物高技术有限公司
第六章 国外主要企业
6.1 罗氏
6.1.1 公司简介
6.1.2 经营情况
6.1.3 营收构成
6.1.4 干扰素业务
6.1.5 在华发展
6.2 默沙东
6.2.1 公司简介
6.2.2 经营情况
6.2.3 营收构成
6.2.4 干扰素业务
6.2.5 在华发展
第七章 总结与预测
1 Overview
1.1 Definition
1.2 Classification
2 Overview of China Interferon Industry
2.1 Development Course
2.2 Status Quo
2.3 Market Size
2.4 Market Structure
2.5 Competitive Landscape
3 China Interferon Market Segments
3.1 Ordinary Interferon
3.1.1 Introduction
3.1.2 Market Size
3.1.3 Competitive Landscape
3.1.4 Price Comparison
3.2 Long-acting Interferon
3.2.1 Introduction
3.2.2 Market Size
3.2.3 Competitive Landscape
3.2.4 Price Comparison
4 Downstream Market of China Interferon Industry
4.1 Hepatitis B
4.1.1 Introduction
4.1.2 Therapy
4.1.3 Interferon Hepatitis B Market Shrinks
4.1.4 Market Size
4.2 Hepatitis C
4.2.1 Introduction
4.2.2 Therapy
4.2.3 The Launch of DAAs Drugs Impacts Chinese Interferon Hepatitis C Market
4.3 Pediatrics
4.4 Others
5 Major Chinese Enterprises
5.1 Anke Biotechnology
5.1.1 Profile
5.1.2 Operation
5.1.3 Revenue Structure
5.1.4 R & D and Investment
5.1.5 Interferon Business
5.1.6 Forecast and Prospect
5.2 Tri-Prime Gene
5.2.1 Profile
5.2.2 Operation
5.2.3 R & D
5.2.4 Major Customers
5.2.5 Interferon Business
5.2.6 Forecast and Prospect
5.3 Kawin Technology
5.3.1 Profile
5.3.2 Operation
5.3.3 Revenue Structure
5.3.4 R & D and Investment
5.3.5 Major Customers
5.3.6 Interferon Business
5.3.7 Forecast and Prospect
5.4 Amoytop Biotech
5.4.1 Profile
5.4.2 Operation
5.4.3 Interferon Business
5.5 Shenzhen Kexing Biotech
5.5.1 Profile
5.5.2 Interferon Business
5.6 Sinobioway Biomedicine
5.6.1 Profile
5.6.2 Interferon Business
5.7 3SBio
5.7.1 Profile
5.7.2 Operation
5.7.3 Interferon Business
5.8 Harbin Pharmaceutical Group Bioengineering
5.8.1 Profile
5.8.2 Operation
5.8.3 Interferon Business
5.9 Changchun Institute of Biological Products Co., Ltd.
5.9.1 Profile
5.9.2 Interferon Business
5.10 Zhejiang Hansheng Pharmaceutical Co., Ltd. of Beisheng Pharma
5.10.1 Profile
5.10.2 Interferon Business
5.11 Others
5.11.1 Hainan Xinmingda Biopharmaceutical Co., Ltd
5.11.2 Beijing Yuance Pharmaceutical Co., Ltd.
5.11.3 Heilongjiang Qingfengyuan Biological Engineering Technology Co., Ltd
5.11.4 Liaoning Satellite Institute of Biological Products Co., Ltd.
5.11.5 Shanghai Chemo Wanbang Biopharma Co., Ltd
5.11.6 Changchun Heber Biological Technology Co., Ltd
5.11.7 Shanghai Huaxin High Biotechnology Inc.
6 Major Foreign Companies
6.1 Roche
6.1.1 Profile
6.1.2 Operation
6.1.3 Revenue Structure
6.1.4 Interferon Business
6.1.5 Development in China
6.2 MSD
6.2.1 Profile
6.2.2 Operation
6.2.3 Revenue Structure
6.2.4 Interferon Business
6.2.5 Development in China
7 Summary and Forecast
表:干扰素分类及其特点
表:干扰素药物的发展历程
图:2010-2016年中国肝病治疗用药市场规模
图:2015年中国肝病治疗用药市场结构
图:2012-2016年中国样本医院干扰素销售额及同比增长率
图:2012-2016年中国样本医院干扰素销售额(分类型)
图:2012-2016年中国样本医院干扰素销售额(分产品)
图:2012-2016年中国干扰素行业市场规模
图:2013-2016年中国干扰素行业市场结构(分类型)
图:2013-2016年中国干扰素行业市场结构(分产品)
图:2015年中国干扰素行业市场份额(分企业)
图:2016年中国干扰素行业市场份额(分企业)
图:2012-2016年中国样本医院普通干扰素销售额
图:2012-2016年中国普通干扰素市场规模
图:2015年中国普通干扰素市场份额(分企业)
表:中国普通干扰素主要企业干扰素产品规格及最新中标价
表:中国长效干扰素注册批准情况
图:2012-2016年中国样本医院长效干扰素销售额
图:2012-2016年中国样本医院长效干扰素销售额(分产品)
图:2012-2016年中国长效干扰素市场规模
图:2012-2016年中国样本医院长效干扰素销售额(分企业)
图:2012-2016年中国长效干扰素市场份额(分企业)
表:2016年中国主要长效干扰素研发企业及其产品研发进度
表:2016年中国长效干扰素企业长效干扰素产品规格及招标价格
图:2013-2016年中国抗乙肝病毒化药物市场规模
表:中国治疗乙肝的主要药物及功能
表:中国主要核苷类药物对比
图:2010-2016年中国样本医院乙肝抗病毒药物份额
图:2013-2016年中国乙肝用干扰素市场规模
图:2008-2016年中国丙肝年发病人数
图:抗丙肝药物的发展历程
表:2013-2016年全球已上市新型抗丙肝药物
表:2016年全球主要在研新型抗丙肝药物
表:2016年中国新型抗丙肝药物申报情况
表:2011-2016年中国艾滋病发病数
图:2011-2016年中国艾滋病死亡人数
表:安科生物发展历程
图:2013-2016年安科生物营业收入及净利润
图:2013-2016年安科生物营业收入(分产品)
图:2013-2016年安科生物营业收入构成(分产品)
图:2013-2016年安科生物研发支出及占总营收比重
表:安科生物干扰素产品
表:安科生物干扰素产品规格
图:2013-2016年安科生物干扰素产品销售收入
图:2016-2021E安科生物营业收入及净利润
图:2013-2016年三元基因营业收入及净利润
图:2013-2016年三元基因研发支出
表:2014-2016年三元基因前五名客户销售金额
表:三元基因干扰素产品规格
图:2015-2021E三元基因营业收入及净利润
图:2013-2015年凯因科技营业收入及净利润
表:2013-2015年凯因科技营业收入构成(分产品)
表:凯因科技在研项目及进展情况
图:2013-2015年凯因科技研发支出
表:凯因科技募投项目
图:2013-2015年凯因科技前五名客户销售收入
表:凯因科技主要干扰素产品
表:凯因科技干扰素产品规格
图:2013-2015年凯因科技干扰素营业收入
图:2013-2015年凯因科技干扰素营业收入(分产品)
图:2015-2021E凯因科技营业收入及净利润
表:特宝生物发展历程
表:2013-2016年特宝生物财务数据
表:特宝生物长效干扰素派格宾简介
表:特宝生物长效干扰素派格宾发展历程
表:深圳科兴生物干扰素赛若金简介
表:未名医药干扰素产品
表:未名医药干扰素产品规格
图:2014-2016年三生制药营业收入及净利润
表:三生制药干扰素产品规格
图:2014-2016年哈药生物营业收入及净利润
表:哈药生物主要干扰素产品
表:哈药生物干扰素产品规格
表:长春生物干扰素产品规格
表:北生药业汉生制药干扰素产品规格
表:欣明达生物干扰素产品规格
表:远策药业干扰素产品规格
表:庆丰源生物干扰素产品规格
表:卫星生物干扰素产品规格
表:凯茂生物干扰素产品规格
表:海伯尔生物干扰素产品规格
表:华新生物干扰素产品规格
图:2013-2016年罗氏营业收入及净利润
图:2013-2016年罗氏营业收入(分部门)
图:2013-2016年罗氏营业收入构成(分部门)
图:2013-2016年罗氏制药部门营业收入(分地区)
图:2013-2016年罗氏制药部门营业收入构成(分地区)
图:2013-2016年罗氏诊断部门营业收入(分业务)
图:2013-2016罗氏诊断部门营业收入构成(分业务)
图:2013-2016年罗氏派罗欣销售额及占总营收比重
图:2013-2015年罗氏派罗欣销售额(分地区)
图:2013-2016年罗氏派罗欣在华样本医院销售额
图:2013-2016年默沙东营业收入及净利润
图:2013-2016年默沙东营业收入(分产品)
图:2013-2016年默沙东营业收入构成(分产品)
图:2013-2016年默沙东营业收入(分地区)
图:2013-2016年默沙东营业收入构成(分地区)
图:2013-2015年默沙东佩乐能销售收入及占总营收比重
表:默沙东在华发展历程
图:2012-2016年默沙东佩乐能在华样本医院销售额
图:2016-2021E中国干扰素行业市场规模
表:2016年中国主要长效干扰素研发情况
图:2016-2021年中国干扰素市场结构(分类型)
图:2016-2021年中国干扰素行业市场规模(分类型)
Classification and Characteristics of Interferon
Development Course of Interferon Drugs
Market Size of Chinese Medicine for Liver Disease Treatment, 2010-2016
Market Structure of Chinese Medicine for Liver Disease Treatment, 2015
Interferon Revenue and YoY Growth Rate of Chinese Sample Hospitals, 2012-2016
Interferon Revenue of Chinese Sample Hospitals (by Type), 2012-2016
Interferon Revenue of Chinese Sample Hospitals (by Product), 2012-2016
China’s Interferon Market Size, 2012-2016
China’s Interferon Market Structure (by Type), 2013-2016
China’s Interferon Market Structure (by Product), 2013-2016
Market Share in China Interferon Industry (by Company), 2015
Market Share in China Interferon Industry (by Company), 2016
Ordinary Interferon Revenue of Chinese Sample Hospitals, 2012-2016
China’s Ordinary Interferon Market Size, 2012-2016
Ordinary Interferon Market Share in China (by Company), 2015
Product Specifications and the Latest Bid Prices of Major Ordinary Interferon Enterprises in China
Registration and Approval of Long-acting Interferon in China
Long-acting Interferon Revenue of Chinese Sample Hospitals, 2012-2016
Long-acting Interferon Revenue of Chinese Sample Hospitals (by Product), 2012-2016
China’s Long-acting Interferon Market Size, 2012-2016
Long-acting Interferon Revenue of Chinese Sample Hospitals (by Company), 2012-2016
China’s Long-acting Interferon Market Share (by Company), 2012-2016
Major Long-acting Interferon R & D Enterprises in China and Their Product R & D Progress, 2016
Product Specifications and Bid Prices of Long-acting Interferon Enterprises in China,2016
Market Size of Anti-Hepatitis B Virus Drugs in China, 2013-2016
Major Drugs for Hepatitis B Treatment and Their Function in China
Comparison between Main Nucleoside Drugs in China
Share of Hepatitis B Antiviral Drugs in Chinese Sample Hospitals, 2010-2016
China’s Hepatitis B-use Interferon Market Size, 2013-2016
China’s Annual Hepatitis C Incidence, 2008-2016
Development Course of Anti-Hepatitis C Drugs
Launched New-type Anti-Hepatitis C Drugs Worldwide, 2013-2016
Main New-type Anti-Hepatitis C Drugs in Research Worldwide, 2016
Application for New-type Anti-Hepatitis C Drugs in China, 2016
China’s AIDS Incidence, 2011-2016
China’s AIDS Death Toll, 2011-2016
Development Course of Anke Biotechnology
Revenue and Net Income of Anke Biotechnology, 2013-2016
Revenue of Anke Biotechnology (by Product), 2013-2016
Revenue Structure of Anke Biotechnology (by Product), 2013-2016
R & D Costs and % of Total Revenue of Anke Biotechnology, 2013-2016
Interferon Products of Anke Biotechnology
Interferon Product Specifications of Anke Biotechnology
Interferon Revenue of Anke Biotechnology, 2013-2016
Revenue and Net Income of Anke Biotechnology, 2016-2021E
Revenue and Net Income of Tri-Prime Gene, 2013-2016
R & D Costs of Tri-Prime Gene, 2013-2016
Revenue of Tri-Prime Gene from Top 5 Clients, 2014-2016
Interferon Product Specifications of Tri-Prime Gene
Revenue and Net Income of Tri-Prime Gene, 2015-2021E
Revenue and Net Income of Kawin Technology, 2013-2015
Revenue Structure of Kawin Technology (by Product), 2013-2015
Ongoing Research Projects and Progress of Kawin Technology
R & D Costs of Kawin Technology, 2013-2015
Fund-raising Investment Projects of Kawin Technology
Revenue of Kawin Technology from Top 5 Clients, 2013-2015
Main Interferon Products of Kawin Technology
Interferon Product Specifications of Kawin Technology
Interferon Revenue of Kawin Technology, 2013-2015
Interferon Revenue of Kawin Technology (by Product), 2013-2015
Revenue and Net Income of Kawin Technology, 2015-2021E
Development Course of Amoytop Biotech
Financial Data of Amoytop Biotech, 2013-2016
Introduction to Amoytop Biotech’s “Paigebin” Long-acting Interferon
Development Course of Amoytop Biotech’s “Paigebin” Long-acting Interferon
Introduction to Shenzhen Kexing Biotech’s “Sairuojin” Interferon
Interferon Products of Sinobioway Biomedicine
Interferon Product Specifications of Sinobioway Biomedicine
3SBio’s Revenue and Net Income, 2014-2016
3SBio’s Interferon Product Specifications
Revenue and Net Income of Harbin Pharmaceutical Group Bioengineering, 2014-2016
Main Interferon Products of Harbin Pharmaceutical Group Bioengineering
Interferon Product Specifications of Harbin Pharmaceutical Group Bioengineering
Interferon Product Specifications of Changchun Institute of Biological Products Co., Ltd.
Interferon Product Specifications of Zhejiang Hansheng Pharmaceutical Co., Ltd. of Beisheng Pharma
Interferon Product Specifications of Hainan Xinmingda Biopharmaceutical Co., Ltd
Interferon Product Specifications of Beijing Yuance Pharmaceutical Co., Ltd.
Interferon Product Specifications of Heilongjiang Qingfengyuan Biological Engineering Technology Co., Ltd
Interferon Product Specifications of Liaoning Satellite Institute Of Biological Products Co., Ltd.
Interferon Product Specifications of Shanghai Chemo Wanbang Biopharma Co., Ltd
Interferon Product Specifications of Changchun Heber Biological Technology Co., Ltd
Interferon Product Specifications of Shanghai Huaxin High Biotechnology Inc.
Revenue and Net Income of Roche, 2013-2016
Revenue Breakdown of Roche (by Division), 2013-2016
Revenue Structure of Roche (by Division), 2013-2016
Revenue Breakdown of Roche’s Pharma Division (by Region), 2013-2016
Revenue Structure of Roche’s Pharma Division (by Region), 2013-2016
Revenue Breakdown of Roche’s Diagnosis Division (by Business), 2013-2016
Revenue Structure of Roche’s Diagnosis Division (by Business), 2013-2016
Roche’s Pegasys Revenue and % of Total Revenue, 2013-2016
Roche’s Pegasys Revenue (by Region), 2013-2015
Roche’s Pegasys Sales in Chinese Sample Hospitals, 2013-2016
Revenue and Net Income of MSD, 2013-2016
Revenue Breakdown of MSD (by Product), 2013-2016
Revenue Structure of MSD (by Product), 2013-2016
Revenue Breakdown of MSD (by Region), 2013-2016
Revenue Structure of MSD (by Region), 2013-2016
MSD’s Peg-Intron Revenue and % of Total Revenue, 2013-2015
Development Course of MSD in China
MSD’s Peg-Intron Sales in Chinese Sample Hospitals, 2012-2016
China Interferon Market Size, 2016-2021E
R&D of Key Long-acting Interferon in China, 2016
Interferon Market Structure (by Type) in China, 2016-2021E
Interferon Market Size (by Type) in China, 2016-2021E
如果这份报告不能满足您的要求,我们还可以为您定制报告,请 留言说明您的详细需求。
|